gold
| properties | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Gold, Au, 79 | |||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | |||||||||||||||||||||||||||||||||||||||
| Group , period , block | 11 , 6 , d | |||||||||||||||||||||||||||||||||||||||
| Appearance | metallic yellow | |||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-57-5 | |||||||||||||||||||||||||||||||||||||||
| EC number | 231-165-9 | |||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.332 | |||||||||||||||||||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.004 ppm | |||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 196,966569 (5) et al | |||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 135 (174) pm | |||||||||||||||||||||||||||||||||||||||
| Covalent radius | 136 pm | |||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 166 pm | |||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Xe ] 4 f 14 5 d 10 6 s 1 | |||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 9.225 554 (4) eV ≈ 890.13 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 20th.203 (25) eV ≈ 1 949.3 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 30th.0 (1.6 eV) ≈ 2 890 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 45.0 (1.7 eV) ≈ 4 340 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 60.0 (1.9) eV ≈ 5 790 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||
| Crystal structure | Cubic area-centered | |||||||||||||||||||||||||||||||||||||||
| density | measured: 19.32 g / cm 3 (20 ° C ); calculated: 19.302 g / cm 3 |
|||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.5 to 3 | |||||||||||||||||||||||||||||||||||||||
| magnetism | diamagnetic ( Χ m = −3.5 10 −5 ) | |||||||||||||||||||||||||||||||||||||||
| Melting point | 1337.33 K (1064.18 ° C) | |||||||||||||||||||||||||||||||||||||||
| boiling point | 3243 K (2970 ° C) | |||||||||||||||||||||||||||||||||||||||
| Molar volume | 10.21 · 10 −6 m 3 · mol −1 | |||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 342 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 12.55 kJ mol −1 | |||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2030 m s −1 | |||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 128 J kg −1 K −1 | |||||||||||||||||||||||||||||||||||||||
| Work function | 5.1 eV | |||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 45.5 · 10 6 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 320 W m −1 K −1 | |||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1, 0, +1, +2, +3 , +5 | |||||||||||||||||||||||||||||||||||||||
| Normal potential | 1.52 V (Au 3+ + 3 e - → Au) | |||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.54 ( Pauling scale ) | |||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||
| NMR properties | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||
Gold (medium high German Golt ; already althochdeutsch gold, to Indo-European root * g h el- , yellow ') is a chemical element with the element symbol Au ( Latin aurum ) and atomic number 79. There is a transition metal and is in the periodic table in the 1 . subgroup (group 11), also known as copper group is referred to. This group contains copper and the precious metals silver and gold. The three metals are also referred to in chemistry as "coin metals". Furthermore, the group also contains the artificially produced, radioactive and extremely short-lived Roentgenium , which has so far had no applications. Gold has been used for ritual objects and jewelry for millennia and since the 6th century BC. In the form of gold coins used as a means of payment. Gold mining has been criticized worldwide for its significant environmental impact.
history
In earlier times gold was found in nature as a solid metal because of its strikingly shiny yellow color . It can be machined very well and does not corrode . Because of the persistence of its luster, its rarity, its apparent immortality and its noticeable heaviness , it was used in many cultures primarily for prominent ritual objects and jewelry.
Prehistory and early history

Gold is one of the first metals to be processed by humans. The gold mining since the early Copper Age proven. The ease of alloying with many metals, the moderate melting temperature and the favorable properties of the alloys made gold very attractive as a material.
The oldest known gold artifacts of mankind are a total of around 3,000 gold objects from the burial ground of Varna (Bulgaria), which were deposited as grave goods and were made between 4600 and 4300 BC. To be dated. More than 7,000 gold objects are from the 4th millennium BC. Known from graves of the Eastern European Maikop culture . The earliest evidence in Central Europe is with the two gold disks in the depository of Stollhof ( Lower Austria ) and also dates from the 4th millennium BC. Since then, gold has been imported from Southeastern Europe in the form of jewelry .
In Central and Northern Europe , golden objects did not appear until the third millennium BC. BC as grave goods, especially in the late Neolithic bell - cup culture . Examples are the earrings and the hair clip at the archer of Amesbury or the gold rings found in 2013 in a bell-shaped grave from Wustermark , Havelland district . Famous examples from the subsequent Bronze Age are the gold plating of the Nebra Sky Disc ( Early Bronze Age ) and the four late Bronze Age gold hats .
The ancient Egyptians exploited deposits in Upper Egypt and Nubia . The location of a gold mine is recorded on the Turin papyrus . The Romans used sites in Asia Minor , Spain ( Las Médulas ), Romania and Germania .
The legend of the voyage of the Argonauts to the Golden Fleece in Colchis was apparently inspired by the sea voyages of Greek prospectors.
The Torah tells of the golden calf , which the Israelites made as an idol while Moses received the Ten Commandments , and of the golden land of Ophir . The New Testament mentions gold (along with frankincense and myrrh ) as one of the gifts of homage from the wise men of the Orient for the newborn Jesus ( Matthew 2:11 EU ).
Gold was processed very early in South America and Mesoamerica . For example, the Mochica in Peru already mastered the formation of alloys ( tumbago ) and gilding at the beginning of the first millennium and made objects for ritual purposes from several kilograms of gold.
The gold extraction and purification was carried out by gold panning , amalgamation and cupellation (oxidizing of base metals with lead , also called refining ) or a combination of the processes.
Modern times

With the supremacy of the European naval powers Spain, Portugal, England and Italy, the greed for gold became a decisive reason for wars and conquests in modern times . Especially the gold wealth of the indigenous peoples in Central and South America attracted European and especially Spanish conquerors ( conquistadores ) after the discovery of America in 1492 , who brought gold to Europe in galleons . For a time Spain became the richest nation in Europe; the indigenous cultures were destroyed by the conquerors or by diseases that were introduced.
Time and again, gold finds attracted large numbers of adventurers. In the 19th century it came on different continents to Gold Rush mass movements mentioned by gold prospectors in the areas of large gold deposits. Examples are the California gold rush in 1849 and the gold rush of 1897 on the Klondike River in Alaska . There was also a gold rush in Australia ( Bathurst , Temora , Teetulpa and Coolgardie ) and South Africa ( Witwatersrand ).
The fluctuating gold price often leads to significant social changes: For example, a falling gold price in South Africa led to a severe impoverishment of the part of the population living on gold mining. In the Brazilian Amazon , the informal gold mining by Garimpeiros is often associated with serious social and ecological consequences.
Occurrence
The gold content in the continental crust is 4 ppb , i.e. about 4 grams per 1000 tons of rock. The proportion varies depending on the region - in deposits that are mined, the gold proportion is often several grams per ton.
Gold occurs predominantly in solid form on earth , i.e. in elementary, metallic form. It is found in primary raw material deposits as gold-bearing rock ( gold ore ) and in secondary deposits, among other things, in soap deposits .
About 43% of the gold mined in 2017 came from the People's Republic of China , Australia , the United States of America, Russia and Canada . The deepest gold mines in the world are located in South Africa. There gold is mined almost 4,000 meters below the earth's surface. At the beginning of 2011, the mining company AngloGold Ashanti was planning shafts up to 5000 meters deep.
In 2016, around 17% of the gold volumes extracted were a by-product of the refining of other metals such as copper , nickel or other precious metals , so that the extraction of gold as a by-product may make the exploitation of other deposits economical.
A total of almost 2700 sites for solid gold have been documented worldwide so far (as of 2017).
Origin of the earthly gold
Most of the elements down to iron, but heavier than hydrogen, were formed in our solar predecessors through nuclear fusions while releasing energy (see also nucleosynthesis ). The gold that occurs on earth - like all elements that are heavier than iron - was created by supernova core collapse while absorbing energy.
Computer simulations carried out in 1994 predicted that when two neutron stars collide, the material ejected into space and the subsequent reaction cascades, along with other heavy elements, would produce around 30 masses of gold. On August 17, 2017, the LIGO detectors recorded gravitational waves that were interpreted as a collision of two neutron stars at a distance of 130 million light years. The reactions of the ejected matter could then be observed with optical telescopes. The measured spectral lines confirmed the prediction that large amounts of gold and other heavy elements were formed during this event.
As long as the early earth did not have a solid crust, all gold moved into the earth's core due to its high density . We only find gold that reached the earth after the crust formation or came back to its surface through volcanic processes.
Primary deposits (mountain gold)
The following sections list some of the main types of primary gold deposits :
Witwatersrand type (paleo-soap deposit)
The Witwatersrand gold field in South Africa is by far the largest in the world. So far, this deposit has delivered more than 40,000 tons of gold. The ore bodies are early Proterozoic (around 1.8 billion years old) paleo- river gravel containing native gold, pyrite and locally minable concentrations of uranium pitchblende . The exact genesis of the deposit is controversial. Classically, the deposit is interpreted as a paleo soap deposit , which means that it falls under the secondary deposits. Around 25% of the gold found has a shape that is typical for transport through hydrothermal solutions, while 75% of the gold is the typical nuggets that speak for fluvial transport. More recent isotope studies, however, suggest a very small-scale hydrothermal mobilization of the gold of a few millimeters to centimeters, so that this gold probably originally came from the river gravel. However, the presence of rounded pyrite and uranium pitch blende clasts definitely indicates that these belonged to the original stock of river gravel. They indicate that the earth's atmosphere at this point in time could only have had a low content of oxygen, since these minerals are not stable under oxidizing conditions.
The resources of the deposit are still tens of thousands of tons of gold, albeit at a considerable depth. Here are the deepest mines in the world (almost 4000 m); mining them is therefore only economical when gold prices are high. The deposit accounts for 40% of the gold plus resources mined worldwide to date.
Gold quartz veins
- Some of the most important gold deposits on earth belong to the orogenic (mesothermal) vein deposits . These deposits mostly occur in metamorphic and deformed marine sediments and magmatites . They arise during mountain formation and are thus tied to old and young fold belts. During mountain formation, metamorphic fluids are released from the rocks involved, which deposit quartz, a little sulphide and gold in crevices . The fluids have a neutral character and temperatures between 250 ° C and 400 ° C. The sulfides are mostly pyrite and arsenopyrite . The gold grades are usually very high, more than 10 g / t is not uncommon. The deposits of this type were formed throughout the history of the earth with significant deposits in the archaic greenstone belts of Africa and Western Australia, during the Proterozoic (USA, Ghana, Brazil), the Palaeozoic deposits of Victoria (Australia) or the young Alpine deposits in the Alps (“Tauern -Gold").
They are mostly pure gold deposits with no possibility of extracting other metals. However, a few deposits contain such high levels of arsenic that they are among the most important deposits of this semi-metal .
- Epithermal gold deposits are closely related to young rock magmatism at subduction zones ( island arches , ocean-continent collisions). Hot hydrothermal fluids from magmas or meteoric waters heated by magmatism transport the gold and deposit it again in corridors, in the form of mineralization or as impregnation in the rock. A distinction is made between “low-sulfidation” and “high-sulfidation” - epi thermal deposits , which are characterized by different fluids and the associated different mineral content. "Low-2sulfidation" deposits are formed from neutral hydrothermal waters with temperatures of 200 to 300 ° C, while "High-sulfidation" deposits are formed from very acidic fluids with over 300 ° C. Both types differ in terms of mineral guidance. Ore grades are usually between 1 and 10 g gold per ton and gold contents from a few 10 to over 1000 t. Some "low-sulfidation" deposits contain large amounts of silver and non-ferrous metals . Recent studies from active hydrothermal fields in New Zealand indicate that large deposits of this type with 1000 t gold content can form in just 50,000 years.
There are significant examples of this type of deposit in Papua New Guinea , New Zealand , Mexico , Peru and Romania, among others .
Carlin type
This type consists of deposits in carbonate rocks. The most important deposits of this type are in Utah and Nevada ( USA ). The deposits there formed in a short interval between 42 and 30 million years ago. They were formed from reduced, moderately acidic fluids with temperatures of 150 to 250 ° C at depths over 2000 m. The ore bodies can contain a few to more than 100 million tons of ore with grades between 1 and 10 g / t. Gold is mostly bound to finely divided arsenic-rich pyrite . As a result, the processing of these ores is relatively complex.
IOCG (Iron-Oxide-Copper-Gold) type
These deposits occur in rockic igneous rocks such as granites and rhyolites . These are large hydrothermal breccia bodies with high levels of iron in the form of hematite and / or magnetite . These deposits were probably formed under a volcanic complex. During an eruption, hydrothermal fluids led to the formation of breccias from igneous rocks and deposited iron oxides, copper sulphides, native gold and other minerals. The most important deposits of this type are in meso Proterozoic rocks of Australia such as Earnest Henry ( Queensland ), Prominent Hill and Olympic Dam (both in the state of South Australia ). The latter represents one of the largest ore bodies on earth with currently assumed resources of 8.4 billion tons of ore. The ore grades are between 0.5 and 2% for copper and 0.5 and 1.5 g / t for gold. Most deposits of this type contain pure copper and gold, while Olympic Dam also contains uranium and silver. This deposit represents the largest known uranium deposit on earth.
Porphyry Cu-Au deposits
Such deposits can be found worldwide in young mountain complexes. These are large ore bodies in intermediate to acidic plutonic magmatites. The ore minerals (pyrite, chalcopyrite , bornite , chalcosine , molybdenite ) occur finely distributed on a network of fissures in the rock. The ore bodies contain tens of millions to several billion tons of ore. The largest deposit of this type is Chuquicamata in Chile with over 10 billion tons of ore. In the USA, Bingham Canyon is the most important deposit and one of the largest gold producers in the country. The ore grades are comparatively low with 0.5 to 1% copper and 0.1 to 1 g / t gold, but the size of the ore bodies allows economic extraction. These deposits are often associated with skarn deposits and epithermal gold deposits can be found in the wider area.
VHMS / SHMS deposits
These deposits form in the marine area. Volcanic Hosted Massive Sulphides (VHMS) are bound to basic igneous rocks (mostly basalts ), while Sediment Hosted Massive Sulphides (SHMS) occur in marine sedimentary rocks. Most of these deposits are pure non-ferrous metal deposits ( lead , zinc , copper ), but some also contain extractable additions of gold, silver and other elements. The Devonian SHMS deposit in Rammelsberg near Goslar in the Harz is the most important German gold deposit with 28 million tons of ore and a gold content of 1 g / t as an admixture to the extremely high lead and zinc content.
Secondary deposit (wash gold / soap gold)
Almost all European rivers carry traces of gold with them. This gold was previously embedded in the rock in the form of mostly small, thin flakes. It is released by the weathering processes of the surrounding rock and gets into the river water and is deposited as river soap .
Small amounts of it, especially tinsel, can be found on the scree banks of the High and Upper Rhine, such as near Istein . These secondary deposits , known as Rheingold , have been washed out in the past centuries and with moderate yields (see also river gold ducats ). The only official gold producer in Germany, a gravel works near Rheinzabern that has belonged to the Holcim Group since 2008 , also uses these deposits.
Promotion worldwide
The world annual production in 2008 was 2,260 tons, in 2011 already 2,700 tons, about a hundred times more than in the 19th century. Currently, more gold is mined in two years than is documented in the thousand years of the Middle Ages combined.
Most of the gold was mined in South Africa for a long time , but its output has been falling since the 1970s. In 2007, Australia produced the largest amount. Since 2008, the largest production volume has come from the People's Republic of China , followed by Australia. The USA has also been producing more gold than South Africa since 2008, and the Russian Federation has produced more gold than South Africa since 2010 .
| Rank 2011 |
country | Delivery rate (in t) | Reserves 2014 7 |
Range (years from 2014) 7 |
||
|---|---|---|---|---|---|---|
| 2007 1 | 2011 4 | 2014 7 | ||||
| 1 |
|
275 | 355 | 450 | 3,000 | 6.7 |
| 2 |
|
246 | 270 | 270 | 9,800 | 36.3 |
| 3 |
|
157 | 200 | 245 | 5,000 | 20.4 |
| 4th |
|
238 | 237 | 211 | 3,000 | 14.2 |
| 5 |
|
101 | 110 | 160 | 2,000 | 12.5 |
| 6th |
|
252 | 190 | 150 | 6,000 | 40 |
| 7th |
|
170 | 150 | 150 | 2,100 | 14th |
| 8th |
|
85 | 90 | 102 | 1,700 | 16.7 |
| 9 |
|
39 | 85 | 92 | 1,400 | 15.2 |
| 10 |
|
84 | 100 | 90 | 2,000 | 22.2 |
| 11 |
|
40 | 55 | 70 | 2,400 | 34.3 |
| 12 |
|
118 | 100 | 65 | 3,000 | 46.2 |
| 13 |
|
65 | 70 | 60 | 1,200 | 20th |
| 14th |
|
42 | 45 | 50 | 3,900 | 78 |
|
|
471 | 630 | 695 | 10,000 | 14.4 | |
| Sum (rounded) | 2,380 | 2,700 | 2,860 | 55,000 | 19.2 | |
There are only a few large gold mining companies in the world whose shares are traded on the stock exchanges. These include Agnico Eagle Mines , AngloGold Ashanti , Barrick Gold , Freeport-McMoRan Copper & Gold, Gold Fields Ltd. , Goldcorp , Kinross Gold , Newmont Mining, and Yamana Gold .
Gold holdings worldwide
In all of human history, an estimated 170,000 tons of gold were mined by 2011 and 190,000 tons by the end of 2017.
190,000 tons of gold had been mined worldwide by the end of 2017. This corresponds to a cube with an edge length of 21 meters (around 8800 cubic meters) of pure gold, and around 24.3 g (a little more than one cubic centimeter) per head of the world population.
Occurrence in Europe
The production of gold in Europe - mostly in Finland and Sweden - is insignificant in an international comparison. The Romanian gold ore deposits are arguably the largest in Europe. In Bulgaria , the disused gold mines Zlata (active mining: 1939–1973) and Krushov Dol (active: 1965–1974) are being explored again. A deposit has been explored in Barsele (in the municipality of Storuman ) in Sweden .
Gold as a mineral
Natural deposits of native gold, i.e. in its elemental form, were known long before the International Mineralogical Association (IMA) was founded. Gold is therefore recognized as a so-called grandfathered mineral as an independent mineral type.
According to the Strunz system of minerals (9th edition) , gold is classified under system no. "1.AA.05" (elements - metals and intermetallic compounds - copper cupalite family - copper group) or in the outdated 8th edition classified under I / A.01 (copper series). The systematics of minerals according to Dana , which is mainly used in English-speaking countries , lists the element mineral under the system no. "01.01.01.01" (gold group).
In nature, gold is usually found in the form of rounded nuggets, as scales or flakes and in dendritic (tree-like) or hair- to wire-shaped aggregates . Gold rarely develops coarsely crystalline steps with octahedral , dodecahedral and cubic crystals. It can work with different minerals associated to be like among other altaite , Ankerit , arsenopyrite , Calaverit , chalcopyrite , krennerite , pyrite , pyrrhotite , quartz , scheelite , Sylvanit (Schrifterz) , tetradymite and tourmaline .
Since gold is an inert element, it usually retains its luster and color and is therefore easily recognized in nature. Nevertheless, it is repeatedly confused with similarly colored minerals such as pyrite ( fool's gold , fool's gold ) and chalcopyrite. Gold is also a component of various types of mineral. Examples of minerals with the highest gold content include bezsmertnovite ((Au, Ag) 4 Cu (Te, Pb); 78.56% Au), tetra-auricupride (CuAu; 75.61% Au), maldonite (Au 2 Bi ; 65.34% Au) and Yuanjiangite (AuSn; 62.40% Au). A total of 33 gold minerals are known to date (as of 2017).
Extraction
Unlike most other metals that comes chemically inert gold usually dignified before and does not have to reduction are extracted from ores, such as iron . It is initially only released mechanically from the surrounding rock. Since gold is not chemically reactive and can therefore only be converted into soluble compounds with difficulty, special processes are used for gold extraction.
Gold that is directly visible without a magnifying glass , so-called “free gold” in the form of nuggets or gold dust, is a rarity. The largest known gold nugget was found in September 2018 by Henry Dole in Australia, with around 2400 ounces (74 kg) of gold. The second largest gold nugget, called "Welcome Stranger", was found in Australia in 1869 and weighed 2284 troy ounces (around 71 kg). Most of the gold in the deposits is finely distributed in tiny particles in the surrounding rock and thus escapes attempts to collect it manually using simple methods.
In practice, several processes are combined with one another in order to obtain the desired high yield. With advances in extraction methods, neglecting the problem of waste and when the market price is high, even mining ore that contains only one gram of gold per ton is worthwhile. Old spoil heaps from former gold deposits are therefore being reconditioned using improved technology.
Gold is a by-product of the refining of other metals and is widely recovered. More than ten percent of the gold mined worldwide is extracted from small-scale mining. It has been estimated that 20% to 30% of the gold mined worldwide is obtained through non-industrial prospecting, i.e. by gold prospectors. Part of it can be viewed as a conflict resource that has a negative impact on the population living there and can lead to the so-called resource curse .
Panning for gold
The so-called gold panning as the simplest process for gold extraction uses the high density of the metal. Gold-bearing sand is slurried with water. Since gold is heavier than the surrounding sand, it settles faster on the ground and can be separated. Gold from river deposits is extracted in this way. Hobby gold prospectors mostly use this method. Its disadvantage, however, is the low yield and the time invested by the searcher. The advantage of this method is the reliable yield of coarse gold particles that are not fully captured by cyanide leaching . It can be improved by introducing pelts into the flowing liquid, in which the smallest gold particles get caught in the fur hair and increase the yield.
Gold panning is sometimes carried out partially mechanically on land or with floating dredgers with integrated washing directly in the river. Ore extracted by mining is mechanically crushed to suitable grain sizes beforehand and the crushed rock is processed in a similar manner.
This process precedes the further exploitation of the gold-bearing sands and silts described below.
Amalgam process
In the amalgam process, the alloy formation between gold and mercury is used to form amalgam . Gold-containing sands and silts are intensively mixed with mercury for gold extraction and purification. The gold, but also any other solid metals such as silver, dissolve in the mercury. Gold amalgam is silver in color; Depending on how much mercury is in excess , it is liquid to pasty, doughy and the melting point of the alloy is lower than that of gold. Due to their high density, amalgam and mercury collect at the bottom of the vessel, the mercury then flows off. By heating the remaining amalgam (as described in detail for fire gilding ) the mercury evaporates and what remains is compact raw gold.
The resulting mercury vapors represent a health hazard (see mercury poisoning ) if they are not captured by a closed distillation system or suction and filtering with activated carbon . Private miners often heat the amalgam in open tin containers using blow torches and other gas burners. The mercury (boiling point 357 ° C) evaporates into the ambient air and immediately condenses. This pollutes the soil and people in the area and the rivers with mercury. The Minamata Convention aims to promote alternatives to the amalgam process.
The amalgam process was already used in ancient times.
Cyanide leach
For larger deposits that allow industrial development, cyanide leaching has been used since the end of the 19th century . Against the background that gold dissolves in an oxygen-containing sodium cyanide solution (sodium salt of hydrocyanic acid HCN) as a complex compound, the metal-containing sands are ground into a dusty layer, and the extraction solution is added in a trickle process with the access of free air. The smallest metal particles are dissolved first because they have the relatively largest reaction surface.
The precious metal is found chemically bound in the highly toxic seepage water . After filtration and precipitation with zinc dust, it is obtained as a brown sludge which, after washing and drying, turns into raw gold by reduction .
This is where the raw gold is cleaned. Refined to fine gold, it is then standardized and ready for the market. The cyanide solutions are reused in circular processes. Nevertheless, hydrogen cyanide and its salts (cyanides) escape into the environment, sometimes in larger quantities, for example in the event of accidents, malfunctions in the system or floods. All of these substances are highly toxic, but easily decomposable. In nature's material cycle, they are relatively quickly degraded by oxidation or decomposed by hydrolysis .
This type of gold extraction leaves enormous spoil heaps and dust with traces of cyanide. Further environmental damage is caused by the uncontrolled discharge of sludge into rivers in countries with little environmental monitoring or sludge settling basins bursting, as in 2000 in Baia-Mare, Romania .
Borax process
A more environmentally friendly process is gold extraction and purification with the help of borax (sodium borate). The addition of borax as a slag-forming flux when melting contaminated gold sets the melting point and viscosity of the melt from oxides and silicates of the accompanying substances ( not gold , as is often the case incorrectly stated). As a result, the melting can be carried out with simpler, inexpensive burners (with the addition of charcoal and extra air supply using a hair dryer and an extension pipe up to the forge or a bellows ), whereby the extraction yield is increased. The gold (or, if silver is present, a gold-silver alloy) settles on the bottom of the melting pan and the oxides float on it. Occasionally other fluxes are added ( e.g. calcium fluoride , sodium carbonate , sodium nitrate or manganese dioxide ). If all gold miners in the world were to use this method, the emission of around 1000 tons of mercury per year could be avoided, which is around 30% of global mercury emissions.
Anode sludge process
Gold is often extracted from anode sludge left over from refining other metals, most notably copper. During the electrolysis , the precious gold is neither oxidized nor dissolved; it accumulates under the anode. In addition to gold, silver and other precious metals are produced, which are separated from one another using suitable processes.
Recovery from residues (recycling)
An important source of the precious metal is the processing of dental and jewelry processing waste as well as old materials containing precious metals, such as selected electronic scrap and electroplating sludge. In 2016, reprocessing provided around 30% of the total gold supply.
In urban sewage sludge , gold is contained in traces that come from the use, processing and wear and tear of gold alloys (abrasion of dental fillings, jewelry chain links, loss and so on). An examination of various samples from Arizona revealed, in addition to various other precious metals, an average content of 0.3 grams of gold per ton of sewage sludge. In 2017, 65 kilograms of gold worth 2.1 million francs were extracted in a slag sorting plant in Switzerland .
In September 2013, Austria's crematoria operators advised on how to deal with the deceased's cremated gold in a legally correct manner, which has so far been handed out to the bereaved in the urn, clumped with bone ash.
Attempts to extract gold from the sea
Fritz Haber tried in the 1920s to extract gold from the sea water, which was used to pay for the German reparations. It was then believed that seawater contained between 3 and 10 milligrams of gold per ton. At 4.4 micrograms of gold per tonne of seawater, the average gold content was around a factor of 1000 lower and clearly too low for economic use. Modern measuring methods have shown that the Atlantic and the northeastern Pacific contain 50–150 femtomoles (fmol) gold per liter of water. This corresponds to 0.010–0.030 µg / m³. In the deep water of the Mediterranean, higher values of 100–150 fmol gold per liter of seawater can be measured. In total, that amounts to 15,000 tons of gold in the world's oceans.
Gold synthesis
The hope of being able to produce gold artificially was cherished by many cultures for centuries. Among other things, the hypothesis of the so-called philosopher's stone , which was supposed to make gold from base metals, arose. The alchemy was sometimes called "artificial illustration of silver and gold," or simply as "alchemy" construed.
For example, in two East Central German manuscripts from the 15th century, a Nikolaus von Paris is mentioned, according to whose alchemical treatise Von silber unde von gold gold can be made by adding ammonia to silver and “red iron”, leaving this mixture in hot horse manure for a week is, then filtered and evaporated to half and with the resulting substance silver can be transmuted into 12-carat gold. If one part of this gold is then mixed with four parts of natural gold, 20-carat gold should result.
In fact, gold is produced in tiny amounts in various nuclear processes ( nuclear fusion or nuclear mission ).
Environmental impact
Since gold is almost only contained in traces in today's mines, 20 tons of rubble are incurred to produce a single gold ring alone, which leads to the considerable destruction of entire landscapes. Considerable quantities of highly toxic mercury , which were washed out during the gold mining process or knowingly released into the environment during evaporation, also permanently poison large areas and rivers. Since gold extraction often has improvisational features and takes place far away from effective official supervision, environmental aspects are often treated as subordinate or ignored.
The negative environmental impacts often lead to conflicts between the prospectors and the local population. However, there are the first projects of ecological gold mining, such as the Oro Verde in Colombia. The fair trade seal was awarded for the first time in February 2011 for bars whose gold comes from this mine . Europe's first suppliers for fair gold were in France and Great Britain, for some time now it has also been available - in addition to non-certified - from ÖGUSSA , which set up its own process line for this.
properties
Physical Properties
Gold consists of only one stable isotope and thus belongs to the 22 pure elements and can easily be alloyed with many metals . The heavy metal is unalloyed as soft as tin with a Mohs hardness of 2.5 to 3 ( VHN 10 = 30–34; containing 44–58 silver).
Due to its unmatched ductility and elasticity, gold can be beaten into wafer-thin gold leaf and rolled into particularly thin foils of around 2000 atomic layers and 100 nanometers thick. This corresponds to only about 1/10 of the wavelength of the red light and results in a translucent film that appears blue-green in transmitted light. Ernest Rutherford used gold foil for his scattering experiment . A 24 km long thread can be drawn from one gram of gold.
Gold crystallizes exclusively in a face-centered cubic space lattice and thus has a cubic closest packing of spheres with the space group Fm 3 m (space group no. 225) . The lattice parameter for pure gold is 0.4078 nm (corresponds to 4.078 Å ) with 4 formula units per unit cell .
However, tests in high pressure research have shown that gold takes on a different structure when it is compressed very quickly and even becomes liquid. During the high pressure tests, small gold samples were compressed extremely strongly within nanoseconds with the help of laser shocks . From 220 gigapascals, the face-centered cubic structure changes into the less compact body-centered cubic structure . When the pressure is increased further to 330 gigapascals, the gold begins to melt. According to the theory of research director Richard Briggs of the Lawrence Livermore National Laboratory , gold is said to have a triple point above about 220 gigapascals at which the face-centered, body-centered and liquid phases can coexist.
Pure gold has a metallic, rich yellow color, which is accordingly known as "golden yellow", and a line color of the same kind . In fine distribution, depending on the grain size, it is yellowish, ocher-brown to purple-purple and is then referred to as gold-purple . With increasing temperature, fine gold loses its color intensity and is light yellow glowing before it melts. The molten metal is lemon yellow, slightly greenish and only regains its intense yellow-orange color when it has cooled down completely. In front of the soldering tube , gold can be easily melted into a perfect ball.
Additions of copper make it appear pink or reddish, lower the melting temperature and at the same time increase hardness, strength and polishability considerably. Increasing amounts of silver change the color of the pure gold from light yellow to light green and finally to white; The melting temperature and hardness change very little. Most metals, including the well-known platinum metals , mercury and ferrous metals , when mixed with increasing proportions, lead to a discoloration in the form of a rather dirty yellow-gray to off-white alloy. The color of gold containing palladium (porpezite) varies between tan and light brown.
Some of the unusual properties such as the golden yellow color and high ductility are currently explained by the influence of relativistic effects on the electron orbitals. The yellowish color is created by absorption in the frequency range of the complementary color blue. The reason for this is the relatively small band gap between the 6s and 5d orbitals due to relativistic effects . While high-energy blue photons are absorbed and lead to electron transitions, the other, less energetic photons (green, yellow, red) are reflected from the spectrum of visible light, which creates the yellow color.
In surface chemistry , various surfaces of Au single crystals and the like are used. a. used in scanning tunneling microscopy (see figure).
The specific enthalpy of evaporation ΔH v of gold is 1.70 kJ / g, significantly lower than that of water (2.26 kJ / g) or iron (6.26 kJ / g, all determined for the boiling point ). In the case of overheated gold melts (as with other melt manipulations, for example in the steel industry ), considerable smoke and evaporation losses can occur if the melting process takes place without sealing or suction and separation in activated carbon .
Chemical properties
Gold is not attacked by common (mineral) acids . Only a few strongly oxidizing acids such as aqua regia (a mixture of hydrochloric acid and nitric acid ) or selenic acid dissolve gold. Tetrachloridoauric acid forms in aqua regia :
The halogens chlorine , bromine and iodine are able to dissolve gold, the latter even in alcoholic solution. In aqueous cyanide solutions , gold is easily soluble as potassium dicyanido aurate (I) under oxidation by oxygen . In hot, acidic hydrothermal solutions , gold is relatively physically soluble. As a result, it is often found in quartz rocks . It has been observed that some humic acids are able to dissolve gold.
use
Around half of the gold traded on the market is processed into jewelry, around a third is acquired by institutional and private investors (excluding central banks), and 9% is used in industry, including dental technology (average values for 2010-2014). Central bank purchases have increased sharply: from 2% of global demand in 2010 to 14% in 2014.
Jewelry, decoration and food additive

Most of the gold extracted is used in the jewelry industry. Goldsmiths process gold and other precious metals into rings, chains, bracelets and other jewelry . The precious metal content is certified by the repunze . Some orders are made of gold ( Order of Kutuzo ). India and China are the two largest markets for gold jewelry, together they account for over 50% of the gold demand in this area.
Gold foil, also called gold leaf , gives non-metallic objects such as picture frames , books ( gold cut ), furniture, figures, architectural elements, stucco and icons the appearance of real gold. Since ancient times, gold leaf has been produced by gold bats from high gold content alloys. Gold is rolled and beaten thinner than the wavelength of visible light. In incident light the foil shines golden yellow, in the back light the light source shines through greenish-blue and shows the impact pattern of the metal. The gilder first prepares the base with an adhesive and then applies the gold foil. Half a square meter of surface can be covered with 1 gram of gold leaf.
Gold has a wide range of decorative uses, for example in galvanic coatings on metals and plastics. Gold pigments can be burned onto porcelain glazes, denture ceramics and glass. Historically, fire gilding of metals with the help of gold-mercury alloys, so-called amalgams, has been proven to be the only useful method in ancient times to produce permanent gilding on silver , bronze or base metals . With the development of galvanic gold plating baths in the late 19th and 20th centuries, this area was qualitatively expanded and replaced.
Gold pigments have historically been used in glass production since the 16th century ( gold ruby glass ), but are largely being replaced by cheaper processes.
In the food sector, gold is used as a food additive E 175 . In the form of gold leaf and gold leaf flakes, it is used to gild dishes, for example for coatings on confectionery and for decorating pralines. It is used in drinks for Danziger Goldwasser and Schwabacher Goldwasser . Metallic gold is considered non-toxic, does not accumulate in the body and is excreted with the rest of the digested food.
Investment and currency
Gold, in the form of gold coins and gold bars, serves as an investment and as an international means of payment. Gold is stored as a currency reserve by many central banks around the world, although the currencies are no longer covered by gold reserves .
Investment gold
Private and institutional investors invest in gold and in securities that track the gold price. In times of crisis ( inflation or economic crisis ) gold is seen as a stable investment that can experience increases in value relative to other investments. Gold has no default risk like most other financial investments, where the interest rate depends, among other things, on the perceived default risk of the market participants. With this consideration, however, it should be noted that the gold price is exposed to strong fluctuations over time.
Gold price

The price of gold is determined on the open market. This has been happening at London Bullion Market since the 17th century . Since September 12, 1919, important gold dealers have been meeting in a Rothschild bank in London to formally fix the gold price (see gold fixing ). Since 1968 there has been another daily meeting at the bank at 3 p.m. London time to set the price again when the US stock exchanges are open. For standardized gold trading on commodity exchanges , “ XAU ” was assigned as a separate currency code according to ISO 4217 . It denotes the price of one troy ounce of gold.
On March 17, 1968, the gold price was split and a two-tier system was introduced. One price could freely adjust to the market, the other was fixed. In 1973 the price of gold was released and possession of gold was permitted again in the United States. China allowed private ownership of gold again in 1983 (see gold ban ).
The gold price depends, among other things, on the current production volumes, the oil price and the rate of the US dollar, since gold is mostly traded in US dollars. It can be influenced by the central banks , which together own around 30,750 tons of gold (as of December 2011), that is almost 19% of the global gold quantity of 170,000 tons.
Gold as currency or currency cover
Historically, gold has been used as a currency for millennia. A monetary unit corresponded to a certain amount of gold. During the German Empire from 1871 to 1918, the legal tender in Germany was the gold mark (see also Kurant coin ), where 2.79 gold marks corresponded to one gram of gold and the Reichsbank exchanged the corresponding amount for physical gold on presentation of a banknote. The gold cover was lifted at the beginning of the First World War; and could not be reintroduced afterwards because of the reparations that swallowed up the gold reserves of the German Empire and because of the multiplication of the amount of paper money put into circulation. This de facto changeover to non-gold-backed money (trust currency or fiat money ) led to the devaluation of the mark during the war and enabled the hyperinflation of the 1920s.
For a long time in the United States, $ 20.67 was the equivalent of an ounce of gold. In 1934, the US dollar was devalued by the redefinition of the gold price to 35 US dollars per troy ounce. The new relationship was confirmed in the Bretton Woods system of 1944.
In order to exclude gold as a currency alternative and to increase the currency reserves ( gold reserve ), gold ownership was temporarily prohibited in the USA. From 1933 to 1973, gold ownership was only allowed in the form of jewelry and coin collections. President Franklin D. Roosevelt had gold confiscated under Executive Order 6102 . President Richard Nixon ended the Bretton Woods system in 1971 and abolished its promise that all national banks could demand one troy ounce of gold for 35 US dollars from the US Federal Reserve.
Since the gold standard limits the amount of money issued and the amount of national debt, governments have been keen to detach their currencies from gold. In both world wars, the gold standard was abandoned because the funds required for war production could only be raised through inflation. Nowadays all currencies in the world are detached from gold, and only then has the extreme expansion of today's money supply and debt been possible. At current rates, the amount of gold available would not be enough to cover the value of a significant currency. The gold available in January 2006 had a market value of € 2.5 trillion and, hypothetically, would have been suitable to cover the national debts of Germany and Spain at the time. In the event of renewed coverage of major currencies, the gold rate would have to rise many times over.
electronics


The electronics industry uses gold and the like. a. due to the good processability and excellent contact (high corrosion resistance, easy solderability):
- Bonding:
- Bonding wires (connecting wires between the chips and the connections of integrated circuits ) as well as bonding islands and conductor structures are partly made of pure gold: one gram can be pulled into a bonding wire that is more than three kilometers long. For reasons of cost, bonding wires made of aluminum or copper are increasingly being used.
- The assembly ( chip bonding ) of microelectronic and laser diode chips takes place on gold-plated surfaces
- Circuit boards (their copper conductor tracks and contact points) with direct connectors are often gold-plated
- Switching contacts for signal switches and relays
- Gold plating of connectors and contact surfaces ("puff gold plating" or up to 1 µm layer thickness)
medicine
Because of its corrosion resistance and aesthetic qualities, it is used in dental prosthetics as a filling or replacement material for defective or missing teeth. Alloys are used because pure gold would be too soft. These usually consist of around 80% gold and 20% secondary metals such as platinum . The popularity of gold teeth has waned in Western countries in favor of less noticeable plastic implants, while they are still widely used in many other parts of the world.
Some gold salts (administered intramuscularly) are used curatively for rheumatism therapy, such as aurothioglucose (aureotan) as a slow-acting long-term therapeutic agent . The gold salts sodium aurothiomalate and auranofin are used as basic drugs against rheumatoid arthritis (chronic polyarthritis). Gold therapies only achieve their full effect after several months and are associated with side effects. It can cause allergic reactions and, if used improperly, damage the liver, blood and kidneys. Around 50% of therapies with gold salts are discontinued due to the undesirable effects. More recently, cheaper drugs with a better side effect profile have replaced treatment with therapeutic agents containing gold.
In 1913 the drug manufacturer Madaus had the homeopathic preparation Essentia Aurea: Goldtropfen patented, which was sold under the brand name "Herzgold" and used against cardiac and general weaknesses.
Since the Middle Ages, gold-plated pills were probably also in use, first among the Arabs. Most of the time, the term “golden pills” ( pillae aureae , pillulae aureae , güldîn körnlîn ) referred to pills made from various ingredients and listed in the Salernitan antidotarium Nicolai , which were offered as “valuable as gold”.
Around 1935 attempts were made to improve the syphilis therapy , which was not very successful before the introduction of antibioca, by using gold preparations.
In the mid-1970s, the American veterinarian Terry Durkes developed gold implantation for pain therapy of arthritic joints in dogs and horses, which has also been used in human medicine as an alternative medical method since 1996 . There is no scientific proof of effectiveness, the procedure is not mentioned in any guidelines.
optics
Gold reflects infrared light very well (98% at wavelengths> 700 nm) and red and yellow light better than blue and violet. For this reason, heat-reflecting coatings on glasses, beam splitters and mirrors - including laser mirrors for lasers in the mid-infrared - as well as on heat protection visors (fire brigade, foundries, etc.) are made from gold layers ( sputtering , vapor deposition , with protective layer).
Gold is a dopant of germanium (germanium gold, Ge: Au for short) - a semiconductor for the detection of infrared from 1 to 8 µm wavelength when cooled to 77 K according to the principle of photoconductivity .
Nanoparticles
Metallic gold particles present in nanoscopic form, i.e. those with a size on the nanometer scale, have recently become the focus of intensive research, because their use as heterogeneous catalysts in organic-chemical reactions enables new, solvent-free processes. This is part of a process of transforming chemical production methods towards green chemistry . Furthermore, gold nanoparticles as an inert carrier material are coated with various molecules, for example for use in a gene gun .
In this context, it was discovered that gold nanoparticles can themselves have chiral structures after adsorption of chiral substances . The chirality of these particles can be controlled by the enantiomers of the adsorbents, but is retained when the process is carried out in an achiral ( racemic ) environment.
Purity and authenticity
Fineness
| carat | Weight per mille of gold in the alloy |
in trade as | Atom% approx. |
|---|---|---|---|
| 24 kt | 999 | Fine gold 999 | 100 |
| 22 kt | 916 2 ⁄ 3 | Gold 916 | 83 |
| 20 kt | 833 1 ⁄ 3 | Gold 833 | 68 |
| 18 kt | 750 | Gold 750 | 50 |
| 14 kt | 583 1 ⁄ 3 | Gold 585 | 38 |
| 10 kt | 416 2 ⁄ 3 | Gold 417 | 23 |
| 9 kt | 375 | Gold 375 | 20th |
| 8 kt | 333 1 ⁄ 3 | Gold 333 | 18th |
The purity of gold is historically given in carats (abbreviated kt). 24 carats correspond to pure gold (fine gold). With the introduction of the metric system, the conversion to per mille was made. The stamp "750" in gold goods means that the metal contains 1000 parts by weight of 750 parts (i.e. 3 ⁄ 4 ) of pure gold, corresponding to 18 carats ("585" corresponds to 14 carats, "375" corresponds to 9 carats and "333" corresponds to) 8 carat). Bullion coins have either 916.6 per mille ( Krugerrand , Britannia , American Eagle ) or 999.9 per mille gold ( Vienna Philharmonic , Maple Leaf , Nugget , American Buffalo ). The purity can be specified with a decimal number, for example as 0.999 or 1.000 (fine gold).
Internationally, high-quality jewelry is usually made of gold alloys with a fineness of 750 or higher. The choice of the fineness used is influenced by regional and cultural preferences. On the American continent, for example, alloys with a gold content of 585 ‰ are used, while in the Middle East rich yellow gold jewelry is particularly valued from a fineness of around 20 to 22 kt (833–916 ‰) and up. In Southeast Asia and in the Chinese, Thai and Malay-influenced cultural area, this traditionally even extends to jewelry made of pure fine gold, which is considered to be of particularly high quality in the local culture.
The proportion of other precious metals ( silver , palladium , platinum , rhodium , iridium, etc.) that may be contained in the stamp is not taken into account.
Gold imitations
Mainly due to the high price of gold, alloys made of base metals have been developed that are used as gold imitation or as a base in the production of doublé . In most cases, these are non-standardized copper alloys with a wide variety of additives. An alloy of at least 50% copper and zinc as the main alloy (up to over 44%) is known as brass . The addition of lead (up to 3%) increases the machinability of the brass. Important types of brass are tombac (over 67% copper) and special brass (contains other metals).
Precious metals are used to make alloys that can appear like gold without gold in them. With some alloys, however, gold is even added in small amounts.
Test methods
The testing of gold for its authenticity and purity and thus the value determination is carried out in principle by three different methods:
- Weighing according to Archimedes' principle : Determination of the specific weight by measuring the displaced water and comparing it with official lists. A simple method that is only accurate with a fine balance. There are also deviations in the case of heavily jagged and irregularly shaped gold pieces.
- Sampling and acid test : Sampling lines are dabbed with sampling acids (mostly nitric acid) in different concentrations. Goldsmiths and coin collectors use this method to get an approximate determination of the fineness in everyday life. During the acid test, part of the test piece has to be rubbed off, so a loss of material is accepted.
- X-ray fluorescence spectrometer : scanning with X-rays in the laboratory and evaluation with a computer program. Very precise determination of the fineness of precious metals without loss of material, but the necessary equipment must be available.
Gold alloys
General
Classic gold alloys for jewelry belong to the three-component system gold-silver-copper. One reason for this is that these metals occur naturally together and until the 19th century it was forbidden in Europe to alloy gold with metals other than copper and silver. The color spectrum of such gold alloys ranges from rich yellow to light green and salmon pink to silver white. These alloys are easy to manufacture and easy to process. Depending on the requirements, the alloy properties can be influenced as desired by adding other metals . For example, small additions of zinc , indium , tin , cadmium or gallium lower the melting temperatures and the surface tension of the metal melt with only a slight change in the color of the alloy. This is a property that makes it suitable for use as solder alloys for other gold materials. Other additives such as platinum , nickel or a higher proportion of copper increase the hardness of the metal mixture considerably, but negatively change the beautiful color of the gold. Additives such as lead (lead-containing solder) , bismuth and many light metals make gold alloys brittle so that they can no longer be deformed.
But not only the type, but also the amount of the added metals changes the gold alloys in the desired way. If a rich natural color is desired, at least three quarters of parts by mass of gold are required for very noble gold alloys. The highest strength and hardness are achieved with the rather paler gold alloys with a fineness of around 585, which is why this empirically found alloy ratio has been used for a long time. Alloys with a significantly lower fineness than these, on the other hand, are threatened by long-term corrosion effects due to the base admixtures .
A further distinction is to be made as to whether the alloys are to be processed as cast material or, as conventionally as wrought alloys , i.e. forgeable, must be suitable for cold forming. The former contain grain refinement additives in the tenths of a thousandth range, which have a beneficial effect on crystal growth when the melt slowly solidifies in the casting mold, while the addition of some silicon suppresses surface oxidation when heated in the air, but worsens the cold workability and solderability.
Alloying in this context ultimately means a “thinning” of the pure gold and its valued properties such as color, corrosion resistance , price, density are “thinned”, but mechanical strength and polishing ability are added.
Precious metal content and corrosion resistance
The user-friendly properties, the “noble quality” of gold alloys, is determined by the ratio of noble metal atoms to the total number of atoms in the alloy. Their properties such as corrosion resistance, color effect or intermetallic bond are determined by this ratio of the number of pieces. The amount of substance, the mole and the stoichiometry indicate this. The weight percentage only indirectly determines the properties and is also very dependent on the additional metals used.
Gold with atomic mass 197 and copper atoms with mass number 63 (only around a third) form an alloy with an atomic ratio of 1: 1. This alloy example shows a weight percentage of 756 parts of fine gold and suggests a high precious metal content via the weight. However, if you look closely, this is only 50% over the proportion of gold atoms (the number of pieces). Empirically, however, an alloy below 50 atomic percent gold can be attacked by acids. The smaller the atomic mass of the alloy additives, the more drastic this effect is.
Viewed in this way, only half of the alloy atoms in the 750 gold alloys commonly used are gold. An extreme example is a 333 gold alloy, here there are only 2 gold atoms for 9 additional atoms. This explains the very base properties of this material, such as a high tendency to tarnish, corrosion behavior and low color depth. Many goldsmiths and countries, such as Switzerland, refuse to regard this alloy as "gold".
Color gold alloys
The figure 750 / ooo - regardless of whether it is white gold, red gold or yellow gold - always means that the same amount of fine gold is contained in the respective alloy. However, copper, silver or palladium and other alloy components change - depending on the color of the gold alloy - in their quantitative composition.
Red gold
Red gold is a gold alloy, consisting of fine gold, copper and, if necessary, a little silver to improve the mechanical workability. The relatively high copper content, which is significantly higher than that of silver, is responsible for the “red” color and hardness of the material, which gives it its name. The color is similar to copper.
Certain gold shades are regionally popular; in the east and south of Europe, for example, darker and more strongly colored reddish gold alloys are used. In the GDR , red gold was colloquially referred to as Russian gold ; Sometimes the term Turkish gold is still in use in southern Germany . Russian gold has the unusual fineness of 583 and is very easy to recognize. The color is a little lighter than that of red gold. Light, pink gold with a low copper content, which can also contain palladium in addition to silver, is offered as rose gold .
Yellow gold
It is a yellow gold alloy similar to fine gold, made of fine gold with silver and copper. The ratio affects the color. As the gold content decreases, the depth of the yellow tone is reduced very quickly. The ratio of the metals added to the gold is usually about 1: 1; the tints and color intensity can be chosen continuously and arbitrarily. The color ranges from light yellow with a clear proportion of silver to yellow-orange with the inverse proportion to the addition of copper. Due to its high recognition value, yellow gold is by far the most popular gold color worldwide.
Green gold
Green gold is a greenish yellow gold alloy with no added copper. The color is created by approximating the atomic ratio gold: silver 1: 1, which in the optimal case corresponds to a gold content of 646, where the clearest shade of green occurs. Since the silver content in this case is already over 40%, the color is relatively light. Up to a third of the silver can be replaced by cadmium , which intensifies the green tone, but reduces the favorable tarnishing properties and the melting temperature. The alloys are very soft and not very strong in color. Green gold is rarely used, usually to represent foliage leaves or the like.
White gold and gray gold
White gold as a collective term denotes gold alloys which, through the addition of clearly discolored additional metals, result in a white-pale gold alloy. The main alloy additives used are the platinum metal palladium , (previously very common) nickel or, if the gold content is low, silver. The discoloration of the naturally yellow gold occurs continuously and requires a certain amount of the discoloring additive; the rest, which is still missing up to the calculated total volume, is often made of copper or silver.
These almost colorless materials were developed in Pforzheim in 1912/13 as a cost-effective and hallmarkable substitute for platinum for jewelry purposes and introduced as white gold in an effective way . In the francophone- speaking area these materials are more appropriately known as or gris , "gray gold". The aim was to produce a tarnish-resistant material that was easy to process and in which colorless diamonds could excellently show their effect. Until now, people were dependent on silver, which darkens, or platinum and the somewhat darker and lighter palladium. Logically, no jewelry with white gold existed before this time.
Many metals form “white” alloys with gold, such as mercury or iron (the alloy with the precious metal gold does not make iron rustproof). Platinum and gold form a heavy, more expensive and very easy to harden alloy. The platinum objects produced in South America in the pre-Columbian period consist of this whitish-beige to dirty-gray-looking material.
White gold containing nickel (a gold-copper-nickel-zinc alloy with a variable 10–13% nickel content) can be viewed as a red gold alloy discolored by the addition of nickel; as a result, it is relatively hard and can be rolled, drawn or forged to spring hardness . The high basic strength enables, for example, lower wall thicknesses with the same stability. Other properties such as excellent machinability and polishability are of great advantage. In addition, there is the low melting point and lower price, which in turn results from the fact that no other precious metals are included in the additive and the density is lower than that of the palladium-alloyed counterpart. For mechanically stressed parts such as brochures, needles, hinges and connecting parts, this material is very much appreciated by jewelry manufacturers and jewelers due to its strength. Nickel white gold is the basis of white gold solder alloys. However, since the nickel content on the skin can cause allergic reactions , it is now largely avoided in almost all modern jewelry alloys.
The more noble alternative is palladium-containing white gold , more appropriately referred to as gray gold . It is comparatively soft, although there are different recipes from hard to soft alloys. These are multi-component alloys with up to six components. The basic color of the palladium-based gold mixtures is generally darker, just "grayer" than that of the nickel-based white gold. The addition of palladium with approx. 13-16% must be chosen higher than with nickel-white gold in order to discolor the overall mixture in a comparable manner. Usually these white / gray gold alloys are usually rhodium-plated after processing . It is therefore less important that the alloy appears completely white or light gray in color, and there is a conscious saving on the addition of palladium, which significantly increases the price and also disadvantageously colors the mixture darker. As a result, these materials often look slightly beige in nature. The comparison with platinum or silver is obvious. The processing properties, such as machinability, which is required for machine turning, for example of wedding rings , place different demands on the tools. The casting properties (higher melting point and higher surface tension of the melt) differ from the nickel-based counterpart. A structural toughness of the alloys increases the effort of high-gloss polishing in an unusual way. The disadvantage is the increased price due to the not inconsiderable proportion of palladium and the higher density of the material. The alloys positively show their high proportion of precious metals (gold-palladium-silver) in their properties. A piece of jewelry made of palladium white gold was around 20% more expensive in January 2007 than a comparable piece made of yellow gold with the same fineness.
Gold alloy suppliers are constantly developing new types of materials. There are white gold alloys with cobalt , chrome , manganese - germanium and other metals. Processing problems, price developments or a lack of customer acceptance often make such new gold alloys quickly disappear from the market.
Since “white” gold cannot be electrochemically deposited, jewelry products made from white gold are usually rhodium-plated by electroplating. This coating with rhodium , a platinum secondary metal, brings about a color improvement to a pure, silver-like white and improved scratch resistance compared to the uncoated metal surface made of pure white gold. This rhodium coating does not have to be specified explicitly. By removing this coating, the actual white or gray gold reappears, which often leads to visual impairments in wedding rings. In recent years, white gold rings have therefore been deliberately sold in their natural color in order to avoid disappointment for consumers.
Titanium-gold alloy
A hardenable titanium- gold alloy with 99% gold and 1% titanium is used in wedding ring production and medical technology. The high proportion of precious metals combined with high strength make the material interesting. The yellow color is comparable to that of 750 yellow gold, but "grayer". Due to the addition of titanium, the alloy is very sensitive when it melts and reacts with oxygen and nitrogen.
links
Gold occurs in its compounds mainly in the +1 and +3 oxidation states . In addition, −1-, +2 and +5-valent gold is known. Gold compounds are very unstable and easily decompose when heated, forming elemental gold.
- Due to the noble character of the element, gold (III) oxide (Au 2 O 3 ) cannot be accessed by combustion with oxygen . Instead, trichloro gold hydrate (AuCl 3 (H 2 O)), which is stable in aqueous solution (as an acid, can actually be referred to as hydrogen trichlorohydroxidoaurate (III) H [AuCl 3 (OH)]), which, mixed with lye, is called gold ( III) hydroxide precipitates. This water splits off on drying and gives gold (III) oxide. Above 160 ° C the oxide breaks down again into the elements.
- Gold (III) chloride (AuCl 3 ) is formed when gold dust is treated with chlorine at approx. 250 ° C or from HAuCl 4 and SOCl 2 . It forms dark orange-red needles that are soluble in water, alcohol and ether. Water decomposes AuCl 3 to hydroxotrichlorogold (III) acid, H [Au (OH) Cl 3 ].
- Tetrachloridogauric acid , H [AuCl 4 ] The tetrahydrate forms lemon-yellow, long crystal needles which dissolve in moist air and which dissolve very easily in water and alcohol; when exposed to light, purple-brown spots appear. HAuCl 4 is formed when the brown-red gold (III) chloride solution is mixed with hydrochloric acid or gold is dissolved in aqua regia and evaporated with hydrochloric acid. It is used in medicine as an etchant as well as in photography (gold tone baths) and in electroplating ( gold plating ). The gold chloride on the market is mostly HAuCl 4 , while the yellow “gold salt” is sodium gold chloride , Na (AuCl 4 ) · 2 H 2 O.
- Gold (I) sulfide and gold (III) sulfide
- Gold cyanide, sodium or potassium dicyanido aurate (I) , (Na or K [Au (CN) 2 ]), which play a role in gold plating and in cyanide leaching. They are obtained by dissolving gold in a potassium or sodium cyanide solution:
- A similar reaction occurs when gold is dissolved in a thiourea solution. Example based on wastewater treatment:
- Cesium auride is an example of gold as an anion with the formal oxidation state −1: CsAu = Cs + Au -
- Gold (V) fluoride is an example of a gold compound that contains gold in the +5 oxidation state.
- Gold (II) sulfate , AuSO 4 , is one of the few compounds with gold in the +2 oxidation state.
- In biology, gold thioglucose is used to experimentally induce obesity in rodents.
Gold compounds can be very toxic due to the toxicity of the connection partner, such as tetrachloridoauric acid and the gold cyanides.
Biological importance
Gold and gold compounds are not essential for living things. Since gold is insoluble in stomach acid, there is no risk of poisoning when consuming pure, metallic gold (e.g. as a decoration). On the other hand, if gold ions accumulate in the body, for example when there is an excessive intake of gold salts, symptoms of heavy metal poisoning can occur. Most plant roots are damaged by the application of (large amounts) of gold salts.
There are people who are allergic to gold alloys (detection attempts using sodium thioaurosulfate are difficult and unsafe). However, this gold allergy is extremely rare and has not yet been adequately investigated. When using gold fillings and other gold-containing dentures, it should be noted that gold alloys contain other components and an allergic effect can usually be triggered by other components, such as zinc .
Metaphorical usage and symbolism
With gold, which stands for valuable and precious, other valuable things are also referred to. Usually an adjective is added, such as "black gold" for oil. Words and idioms in which gold appears are usually positive or euphemistic in their meaning .
Examples:
- Black gold - oil , coal , tires (racing), caviar , shakudō , coffee , slaves , truffles
- White gold - marble , table salt , cocaine , cotton , porcelain , ivory , asparagus
- Blue gold - drinking water (in arid areas)
- Red gold - wine , saffron , tomato
- Green gold - sugar cane , jade
- Liquid gold - honey , whiskey , beer
- Gold of the sea (sea gold) - corals
- Gold of the north - amber
- Ackergold - potato
- Fool's gold or fool's gold - pyrite
- Trumpet gold - a joke term for brass
- Nose gold - cocaine , nasal secretions ( booger )
- Hüftgold - high-calorie food or fat pads on the body
- Concrete gold - real estate
- spot on - absolutely spot on
- earn a golden nose - be financially very successful in business
- Golden October - mild, sunny weather period in October of each year, so called because of the golden yellow foliage .
- Heart made of (pure) gold - a trait that is characterized by care, humanity and sacrifice.
- have golden hands - be particularly gifted with craftsmanship
- golden mean , golden mean - compromise solution
- Golden ratio - harmonious division of a line, rectangle with harmonious aspect ratio
- Golden wedding
There are counterexamples to these positive expressions, for example, golden water taps are not only symbols of great luxury, but also symbols of decadence . "Blood gold" was used to describe illegally exported amounts of gold during the Second Congo War , with which the militias involved financed their weapons purchases (see also → Blood diamonds ).
Scrap collectors refer to copper as “gold” because they achieve the highest price of the common metals for copper.
heraldry
The heraldic designation "gold" stands for yellow (like "silver" for white). In heraldry, yellow and white are called "metals" and should, if both appear in the same coat of arms, be separated from each other by a "color" (such as red, blue, green, black) (see tinging ).
See also
- Gold / tables and graphics (statistical and geographic data)
- Colloidal gold (solution or gel with tiny gold particles, colored deep red)
- Gold electrolyte
literature
- Andrej V. Anikin : Gold . 3rd, newly written and expanded edition. Verlag Die Wirtschaft, Berlin 1987, ISBN 3-349-00223-4 .
- 5000 years of gold and ceramics from Africa . Heinrich-Barth-Verlag, Cologne 1989, DNB 211467049 .
- Harry H. Binder: Lexicon of the chemical elements - the periodic table in facts, figures and data . Hirzel, Stuttgart 1999, ISBN 3-7776-0736-3 .
- Eoin H. Macdonald: Handbook of gold exploration and evaluation . Woodhead, Cambridge 2007, ISBN 978-1-84569-175-2 .
- Thorsten Proettel: The most important things about gold investments, investment advice . Sparkassen Verlag, Stuttgart 2012.
- Hans-Jochen Schneider: Gold in America. In: The Geosciences. Volume 10, No. 12, 1992, pp. 346-352. doi: 10.2312 / geosciences . 1992.10.346 .
- Christoph J. Raub, Esther P. Wipfler: Gold (material) . In: RDK . Laboratory (2014).
- Bernd Stefan Grewe: Gold. A world history (= CH Beck Wissen. Volume 2889). CH Beck, Munich 2019, ISBN 978-3-406-73212-6 .
Web links
- Material archive: Feingold - Extensive material information and pictures
- Mineral Atlas: Gold - Gold as a mineral
- Where does our gold come from? from the alpha-Centauri television series(approx. 15 minutes). First broadcast on Dec 3, 2000.
Individual evidence
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Geophysics, Astronomy, and Acoustics; Abundance of Elements in the Earth's Crust and in the Sea, pp. 14-18.
- ↑ The values for the properties (info box) are taken from www.webelements.com (gold) , unless otherwise stated .
- ↑ IUPAC, Standard Atomic Weights Revised v2 ( Memento of March 3, 2016 in the Internet Archive )
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on gold in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e entry on gold at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1509.
- ↑ a b c gold . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 57 kB ; accessed on January 11, 2018]).
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics. CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . Volume 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics . Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ↑ a b Entry on gold in the GESTIS substance database of the IFA , accessed on April 25, 2017 (JavaScript required)
- ↑ Kluge. Etymological dictionary of the German language . Edited by Elmar Seebold . 25th, revised and expanded edition. De Gruyter, Berlin / Boston 2011, p. 366.
- ↑ Tom Higham et al. a .: New perspectives on the Varna cemetery (Bulgaria) - AMS dates and social implications . In: Antiquity Journal . tape 81 , no. 313 . New York 2007, p. 640-654 .
- ^ Svend Hansen: Gold and silver in the Maikop culture. In: Metals of Power - Early Gold and Silver. Abstracts of the 6th Central German Archaeological Day, October 17-19, 2013. (PDF) .
- ^ Silke Schwarzländer: Oldest gold in Brandenburg. Rich special burial of the bell beaker culture in Wustermark, Havelland district. In: Archeology in Berlin and Brandenburg . tape 2004 . Konrad Theiss Verlag, Darmstadt 2005, p. 34-35 .
- ↑ Neolithic gold: exceptional finding during bridge construction in Wustermark, district Havelland. (No longer available online.) Brandenburg State Office for Monument Preservation, archived from the original on August 16, 2014 ; accessed on December 2, 2014 .
- ↑ Alfred Grimm , Sylvia Schoske : The secret of the golden coffin: Akhenaten and the end of the Amarna period. Munich 2001.
- ↑ United States Geological Survey: World Mine Production and Reserves January 2017
- ↑ Extreme gold prospecting: South Africans want to descend 5000 meters. on: goldreporter.de , February 18, 2011.
- ↑ MinEx Consulting: Long term trends in gold exploration , p. 18.
- ↑ Find location list for solid gold in the Mineralienatlas and Mindat
- ↑ Matthias Oppliger: One big bang and then there are worlds of gold. In: TagesWoche. December 24, 2017. (tageswoche.ch)
- ^ WEL Minter, M. Goedhart, J. Knight, HE Frimmel: Morphology of Witwatersrand gold grains from the Basal Reef; evidence for their detrital origin. In: Economic Geology. Volume 88, No. 2, April 1993, pp. 237-248 doi: 10.2113 / gsecongeo.88.2.237 .
- ↑ Hartwig E. Frimmel, WE Lawrie Minter, John Chesley, Jason Kirk, Joaquin Ruiz: Short-range gold mobilization in palaeoplacer deposits. In: Mineral Deposit Research: Meeting the Global Challenge. 2005, pp. 953-956, doi : 10.1007 / 3-540-27946-6_243 .
- ^ HE Frimmel: Earth's continental crustal gold endowment: Earth Planet. In: Sci. Letters. Volume 267, 2008, pp. 45-55.
- ↑ Holcim Kies und Beton GmbH acquires new gravel works and a dry sand works. ( Memento from June 28, 2012 in the Internet Archive ) Press release Holcim-Süd. April 1, 2008, accessed August 23, 2012.
- ↑ Christoph Seidler: Treasure hunters raise the Rhine gold. In: Spiegel Online. 23 August 2012. Retrieved the same day.
- ^ US Geological Survey, Gold Statistics and Information , accessed Oct. 26, 2012.
- ↑ World Gold Council FAQ: How much gold has been mined? The best estimates available suggest that the total volume of gold ever mined up to the end of 2009 was approximately 165,600 tonnes, of which around 65% has been mined since 1950. (No longer available online.) Archived from the original on December 5, 2010 ; accessed on December 2, 2014 .
- ↑ a b Thorsten Proettel: The most important thing about gold investments, investment advice. Sparkassen Verlag, Stuttgart 2012.
- ↑ a b Thomas Jüstel: Chemistry Records (PDF file). Accessed June 2020
- ↑ Infographic: Gold Bars .
- ↑ Euromax Ressources: Technical Report on the Gold Resources at Trun Property - Trun and Breznik Municipalities, Pernik District, Bulgaria. ( Memento of April 10, 2014 in the Internet Archive ) (PDF; 5.7 MB).
- ^ Northland: Barsele Rapidly-Growing Gold Resource. ( Memento of February 14, 2009 in the Internet Archive ).
- ^ IMA / CNMNC List of Mineral Names; September 2017 (PDF 1.67 MB; gold see p. 76)
- ^ IMA / CNMNC List of Mineral Names; 2009 (PDF 1.8 MB, gold see p. 109).
- ↑ Webmineral - Minerals Arranged by the New Dana classification. 01/01/01 Gold group .
- ↑ Webmineral - Mineral Species sorted by the element Au (gold)
- ↑ Edelmetalle - Gold ( Memento of April 9, 2011 in the Internet Archive ) (PDF; 197.1 kB, p. 4).
- ↑ Biggest gold find of all time. In: Hielscher or Haase. Deutschlandfunk Nova, September 12, 2018, accessed on April 27, 2020 .
- ↑ Shannon Venable: Gold: A Cultural Encyclopedia. ABC-CLIO, 2011, ISBN 978-0-313-38431-8 , p. 118.
- ↑ Illegal prospectors: Expensive gold is destroying the rainforest. In: Spiegel online. April 20, 2011.
- ^ Justus Freiherr von Liebig , Johann Christian Poggendorff , Friedrich Wöhler (ed.): Concise dictionary of pure and applied chemistry . Friedrich Vieweg and Son, Braunschweig 1842 ( limited preview in the Google book search).
- ↑ See the gold-mercury phase diagram in: H. Okamoto, TB Massalski: The Au-Hg (Gold Mercury) System. In: Bulletin of Alloy Phase Diagrams. 1989, doi: 10.1007 / BF02882176 .
- ^ Chemicals and Waste Branch UNEP: ASGM: Eliminating the worst practices . YouTube, September 2017.
- ↑ gold extraction ( Memento from August 16, 2013 in the Internet Archive ), English, at geology.com
- ↑ Peter WU Appel, Leoncio Na-Oy: The Borax Method of Gold Extraction for Small-Scale Miners . In: Journal of Health and Pollution . tape 2 , no. 3 , 2012 ( journalhealthpollution.org [PDF; accessed December 2, 2014]).
- ^ John O. Marsden, C. Iain House: The Chemistry of Gold Extraction. 2nd Edition. Society for Mining, Metallurgy and Exploration, 2006, ISBN 0-87335-240-8 , p. 455 (partly available from Google Books) .
- ↑ Borax replacing mercury in small-scale mining PDF file ( Memento from February 18, 2015 in the Internet Archive ).
- ↑ Walter A. Franke: Quick assays in mineral identification A guide to experiments for mineral collectors and geoscientists in field work. ( PDF file , English).
- ↑ See the videos at Mercury-free gold mining ; at appelglobal.com.
- ^ A b Filipino Gold Miner's Borax Revolution ( October 13, 2016 memento in the Internet Archive ), Blacksmith Institute website , March / April 2012.
- ^ John O. Marsden, C. Iain House: The Chemistry of Gold Extraction. 2nd Edition. Society for Mining, Metallurgy and Exploration, 2006, ISBN 0-87335-240-8 , p. 457.
- ^ World Gold Council: Gold Demand Trends
- ↑ Jan Dönges: Sewage sludge contains gold for millions of euros. In: Spectrum of Science Online. January 20, 2015, accessed November 5, 2016 .
- ↑ Güsel is worth gold - KEZO is the «Mecca of Swiss metal recycling». In: srf.ch . January 30, 2018, accessed February 1, 2018.
- ↑ Crematoria advise on dental gold. ORF.at, September 4, 2013.
- ↑ Ralf Hahn: Gold from the sea. The research of the Nobel Prize winner Fritz Haber in the years 1922–1927. 1999.
- ↑ Dietrich Stoltzenberg: Gold from the sea? - Fritz Haber's work on the gold content in sea water. In: Chemistry in Our Time . Volume 28, No. 6, 1994, pp. 321-327, doi: 10.1002 / ciuz.19940280611
- ^ Reinhard Osteroth: Economic history: Money in need? Gold from the sea! In: The time . No. 35/2011 ( online ).
- ↑ K. Kenison Falkner, J. Edmond: Gold in seawater. In: Earth and Planetary Science Letters. Volume 98, No. 2, 1990, pp. 208-221, doi: 10.1016 / 0012-821X (90) 90060-B .
- ↑ Ernst von Meyer : History of Chemistry . 1914.
- ↑ Lotte Kurras: Nicholas of Paris. In: Author's Lexicon . Volume VI, Col. 1128.
- ↑ Dangerous gold mining: "A wedding ring produces 20 tons of toxic waste". In: Spiegel online. March 20, 2008. (spiegel.de)
- ↑ Mineria Aurifera de Madre de Dios y contaminación con Mercurio. (PDF; 10.0 MB) (No longer available online.) In: Ministry of Environment Peru. 2011, archived from the original on May 9, 2012 ; Retrieved August 26, 2012 (Spanish).
- ↑ Dangerous mercury when panning for gold. on: derstandard.at , March 5, 2009.
- ↑ Gold mining threatens people and the environment. Rainforest Save , March 19, 2008.
- ↑ Eco-Gold: English gold bars receive the environmental label for the first time. Gold reporter
- ^ Ralph WG Wyckoff: Crystal Structures . 2nd Edition. tape 1 . John Wiley & Sons, New York / London, Sydney 1963, p. 3 (in the appendix ).
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 34 .
- ↑ New structure of gold discovered. Abrupt compression first loosens the atomic lattice of gold, then even makes it liquid. In: scinexx.de. scinexx das wissensmagazin, August 5, 2019, accessed on September 27, 2019 .
- ^ Friedrich Klockmann : Klockmanns textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 395 (first edition: 1891).
- ↑ porpezite , Mindat.
- ↑ Physics Journal. February 2017, p. 19.
- ^ Relativity in Chemistry. Math.ucr.edu, accessed April 5, 2009 .
- ↑ Hubert Schmidbaur, Stephanie Cronje, Bratislav Djordjevic, Oliver Schuster: Understanding gold chemistry through relativity . In: Chemical Physics . tape 311 , no. 1–2 , 2005, pp. 151–161 , doi : 10.1016 / j.chemphys.2004.09.023 , bibcode : 2005CP .... 311..151S .
- ↑ Chemistry: Cracks in the Periodic Table. In: Spektrum.de. Retrieved January 19, 2019 .
- ↑ JV Barth, H. Brune, G. Ertl, RJ Behm: Scanning tunneling microscopy observations on the reconstructed Au (111) surface: Atomic structure, long-range superstructure, rotational domains, and surface defects. In: Phys. Rev. B . Volume 42, 1990, pp. 9307-9318, doi: 10.1103 / PhysRevB.42.9307 .
- ↑ Legal limitation of wastewater emissions from the production and processing of precious metals and the production of mercury metal, at Lebensministerium.at, (PDF file) ( Memento of March 7, 2014 in the Internet Archive ), p. 4.
- ^ Arnold Holleman, Egon Wiberg: Textbook of Inorganic Chemistry. 91-100, verb. and strong exp. Edition. Walter de Gruyter, Berlin / New York 1985, ISBN 3-11-007511-3 .
- ↑ Etching of gold with KI / I 2
- ↑ Yongfeng Zhu, Fang An, Juanjuan Tan: Geochemistry of hydrothermal gold deposits: A review. In: Geoscience Frontiers. 2, 2011, p. 367, doi: 10.1016 / j.gsf.2011.05.006 .
- ^ Brian J. Alloway: Heavy metals in soils, analysis, concentration, interactions Springer-Verlag, 1999, ISBN 3-642-63566-0 , p. 341, doi: 10.1007 / 978-3-642-58384-1 .
- ↑ Gold demand gold.org (English). For the percentages, see the subsections Jewelery, Investment, Central Banks, Technology .
- ↑ Gold demand: Jewelery gold.org (English)
- ↑ Entry on E 175: Gold in the European database on food additives, accessed on June 16, 2020.
- ↑ World Gold Council: World Official Gold Holdings, December 2011.
- ↑ Thorsten Proettel: The most important thing about gold investments, investment advice. Sparkassen Verlag, Stuttgart 2012, pp. 7 and 24.
- ↑ PCB surfaces , accessed in September 2015.
- ↑ Wolfgang Miehle: Joint and spinal rheumatism. Eular Verlag, Basel 1987, ISBN 3-7177-0133-9 , p. 77 f.
- ↑ Information on the Herzgold trademark in the register of the German Patent and Trademark Office (DPMA)
- ^ CJS Thompson: The dawn of medicine. A chapter in the history of pharmacy from the earliest times to the tenth century. In: Janus. Volume 28, 1924, pp. 425-450, here: p. 448.
- ↑ Gundolf Keil: "blutken - bloedekijn". Notes on the etiology of the hyposphagma genesis in the 'Pommersfeld Silesian Eye Booklet' (1st third of the 15th century). With an overview of the ophthalmological texts of the German Middle Ages. In: Specialized prose research - Crossing borders. Volume 8/9, 2012/2013, pp. 7–175, here: pp. 18, 21 and 43.
- ↑ Paul Diepgen : Gualtari Agilonis Summa medicinalis. According to the Munich Cod. La. Nos. 325 and 13124 edited for the first time with a comparative consideration of older medical compendia from the Middle Ages. Leipzig 1911, p. 72 (pillul [a] e aure [a] e) .
- ↑ W. Heuck: The gold therapy, a necessary complement to the recent syphilis therapy, negotiations of the German Dermatological Society 17th Congress held in Berlin 8th-10th October 1935. In: Arch. Dermatol. Syph. Volume 172, 1935, pp. 75-79.
- ↑ TE Durkes: Gold bead implants . In: Probl Vet Med . No. 4 , 1992, pp. 207-211 , PMID 1581658 .
- ^ A. Larsen, M. Stoltenberg, G. Danscher: In vitro liberation of charged gold atoms: autometallographic tracing of gold ions released by macrophages grown on metallic gold surfaces . In: Histochem Cell Biol . tape 128 , 2007, pp. 1-6 , PMID 17549510 .
- ↑ File: Image-Metal-reflectance.png , M. Bass, EW Van Stryland (Ed.): Handbook of Optics. 2nd Edition. vol. 2, McGraw-Hill, 1994, ISBN 0-07-047974-7 .
- ↑ C. Couto, R. Vitorino, AL Daniel-da-Silva: Gold nanoparticles and bioconjugation: a pathway for proteomic applications. In: Critical reviews in biotechnology. [Electronic publication before going to press] February 2016, doi: 10.3109 / 07388551.2016.1141392 . PMID 26863269 .
- ↑ Pablo D. Jadzinsky, Guillermo Calero1, Christopher J. Ackerson, David A. Bushnell, Roger D. Kornberg: Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution. In: Science. Volume 318, 2007, pp. 430-433; doi: 10.1126 / science.1148624 .
- ↑ Cyrille Gautier, Thomas Bürgi: Chiral Inversion of Gold Nanoparticles. In: J. Am. Chem. Soc. Volume 130, 2008, pp. 7077-7084, doi: 10.1021 / ja800256r .
- ↑ Jochem Wolters: The gold and silversmith. Volume 1: Materials and Materials. 2nd, revised edition. Rühle-Diebener-Verlag, Stuttgart 1984, chapter 1.4.8 Copper and its alloys .
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 .
- ↑ MS Wickleder: AuSO 4 , a true gold (II) sulfate with an Au 2 4+ cation. In: Z. Anorg. General Chem. Volume 627, 2001, pp. 2112-2114 doi : 10.1002 / 1521-3749 (200109) 627: 9 <2112 :: AID-ZAAC2112> 3.0.CO; 2-2
- ↑ Everything about allergology - gold .
- ↑ Blood gold from the Congo. In: Spiegel online. accessed on January 8, 2014.

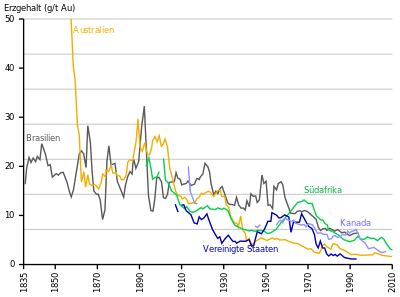













![{\ displaystyle {\ ce {2Au + H2O + 1 / 2O2 + 4NaCN -> 2Na [Au (CN) 2] + 2NaOH}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ec157dd1cfa23c50e477edc56b74f5f771451567)
![{\ displaystyle {\ ce {2Na [Au (CN) 2] + Zn -> Na2 [Zn (CN) 4] + 2Au}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/257e589e015d6284d6274da0158a55d09e72feb5)





![{\ displaystyle {\ ce {2Au + 1 / 2O2 + H2O + 4KCN -> 2K [Au (CN) 2] + 2KOH}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/11d7d454b710143af9dc08cd8c6dc29c9228c90b)
![{\ displaystyle {\ ce {2Au + 4 {Thiourea (TH)} + Fe2 (SO4) 3 -> [Au (TH) 2] 2SO4 + 2FeSO4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/338f74a7d8f48e73ced0f7cd3c42f55602e55b42)